Welcome to Chemistry 250: Structures of Organic Molecules!
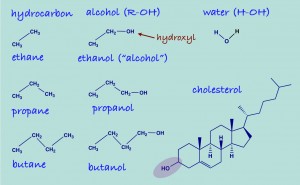
We have hit the ground running full speed ahead in Orgo 1. Already students have dived into the first three (yes 3 !) chapters of their text books. They are learning how to draw organic molecules and identify different functional groups. Functional groups are characteristic groups of bonded atoms that have predictable properties. For example, alcohols are one type of functional group. They consist of an oxygen atom and a hydrogen atom bonded together at the end of a chain of carbons atoms. Wood alcohol (methanol), alcohol for consumption (ethanol), rubbing alcohol (iso-propyl alcohol) and even cholesterol all have this C—O-H bond.
The students will begin their lab work tomorrow by extracting caffeine molecules from tea leaves. During this lab they will learn basic organic techniques such as separation, filtration, recrystallization and melting point determination. This week students will also be utilizing infrared spectroscopy. IR spectroscopy is a useful tool for identifying functional groups on organic molecules. Molecules are subjected to wavelengths of infrared light and different types of bonds will absorb the light and be excited in different ways.
If you want to learn all of the ways bonds exhibit vibrational excitation come stop by the lab at 1 on Wednesday for our awesome IR dance!